When a new development cycle begins for a device meant to monitor a patient’s respiratory condition, manufacturers should consider the challenges their design will face with humidity. Humidity is a catalyst for disruption, as it increases the risk of costly device damage and affects analysis of patients’ respiratory state. Too much moisture in the device can cause equipment wear and skew analyte measurement, making it difficult for clinicians to properly monitor their patients. Perma Pure offers a product that effectively and efficiently removes water vapor from the gas stream, drying the sample, and reducing risk of condensation entering your device. Our FDA cleared product, the ME Dryer, is built with Nafion™ tubing, a highly selective polymer, with chemical properties that make our dryer a proven method of controlling humidity.

How do ME Dryers, built with Nafion™ tubing, remove humidity from breath?
Primarily adopted in respiratory devices, our ME dryers are a proven solution to humidity challenges faced by medical OEMs. ME Dryers are made from Nafion™ tubing, a semi-permeable copolymer of Teflon™ that controls moisture through a first order kinetic reaction. ME Dryers are biocompatible and protected by braided polypropylene monofilament, decreasing risk of tubing damage or kinking. The dryers remove up to 90% of moisture in breath samples and achieve moisture transfer within milliseconds. When a patient exhales into the air, their breath typically has a relative humidity of 100% and a temperature of 37 degrees Celsius. In these conditions, as the breath sample cools down and goes towards the device, the water vapor in the sample would turn into condensate, enter the device interface, and negatively affect device performance. Since cooling begins the second the patient exhales and the breath enters ambient conditions, enough moisture must be removed quickly, while maintaining the analytes in the sample necessary for patient assessment
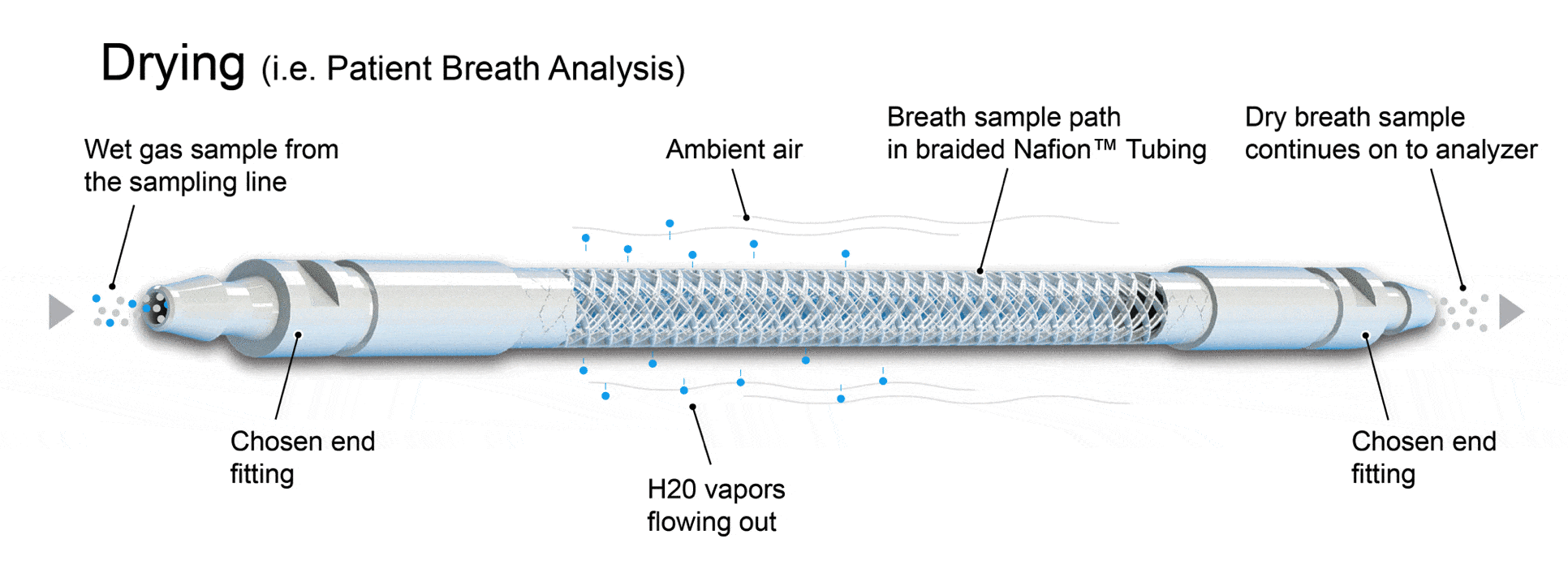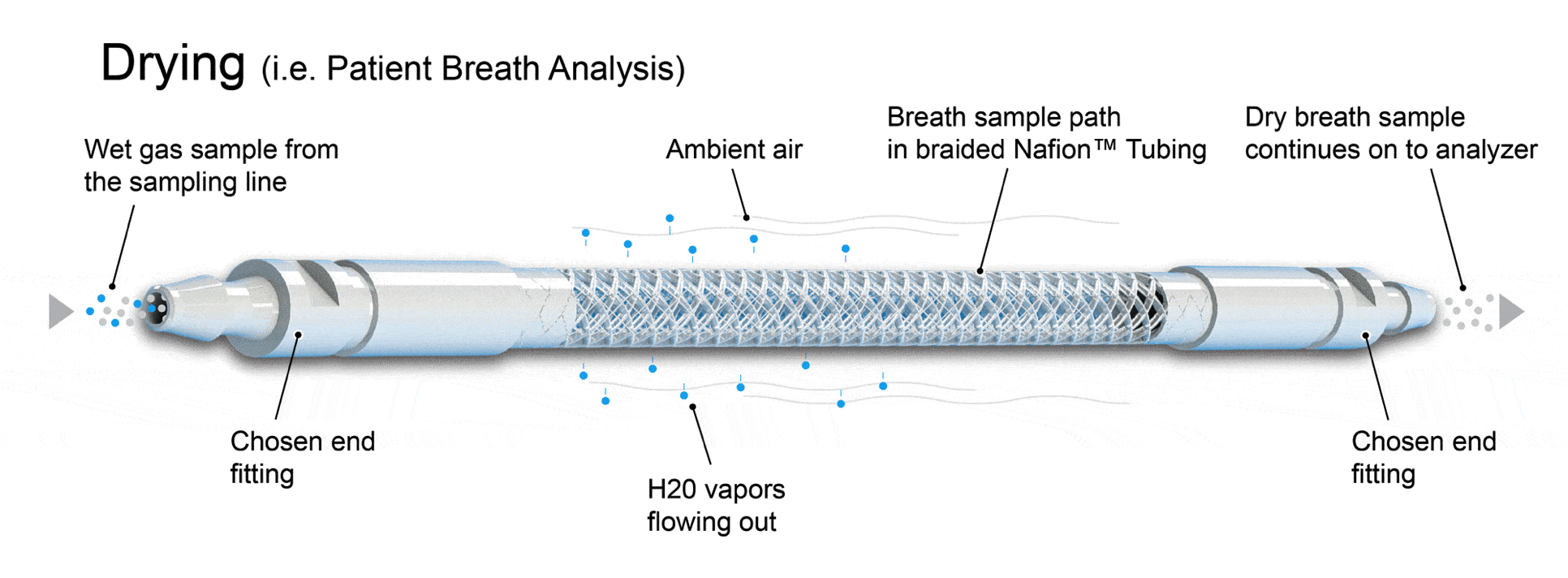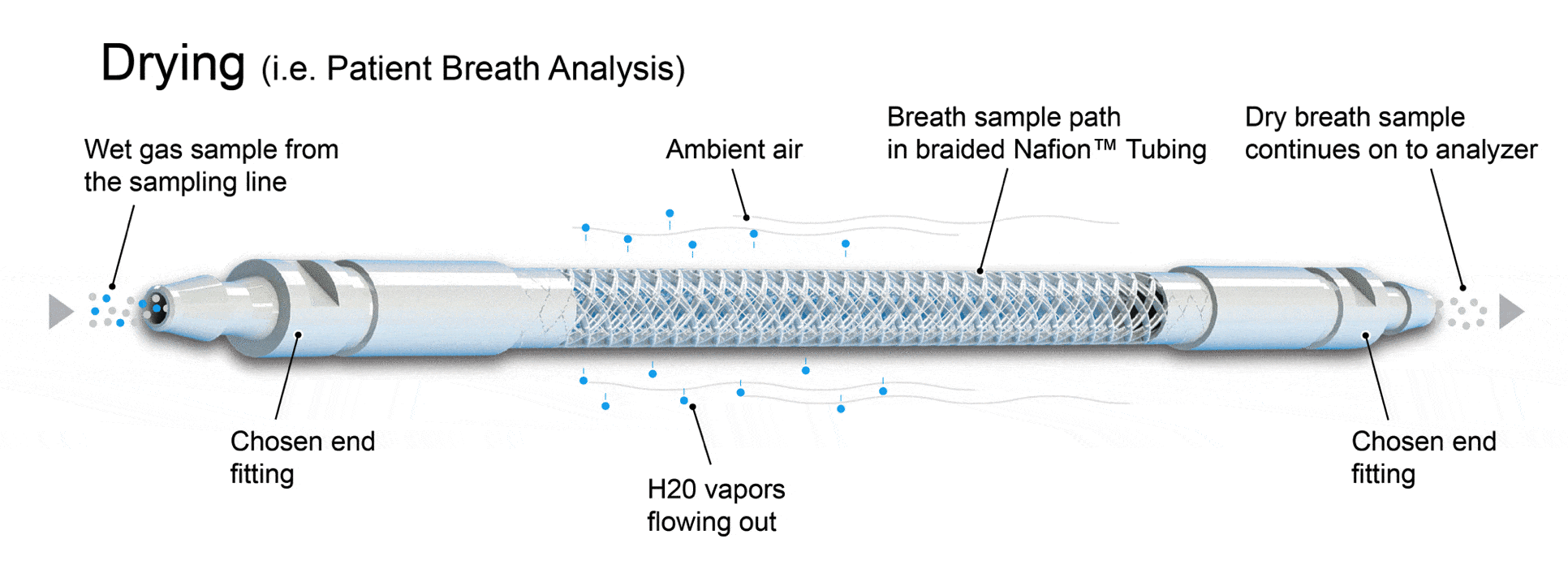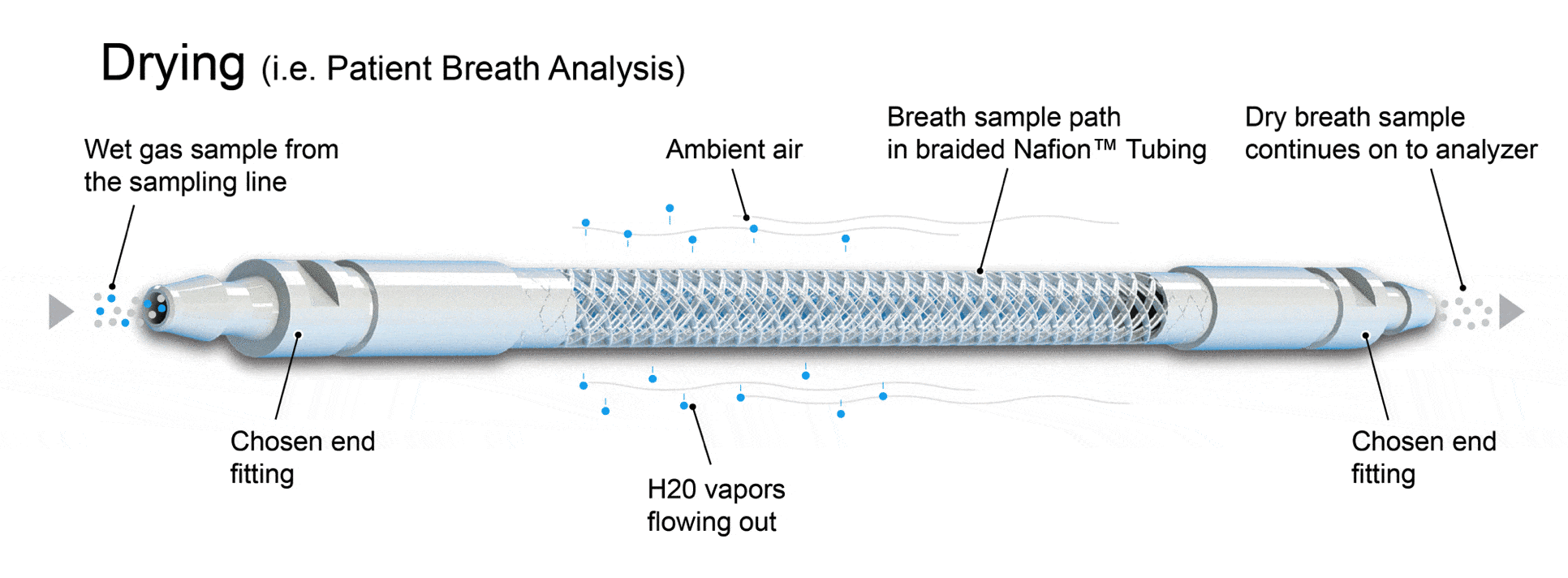
When the breath sample enters the Nafion™ tubing, H2O particles are removed via ion channels in the Nafion™ tubing walls. Since our ME Dryers are highly selective, analytes, including critical oxides, will be retained in the breath sample. This transfer continues as the sample flows through the ME Dryer membrane until the partial pressure of water vapor in the areas surrounding the tubing equates to the pressure of water vapor inside the tubing. This means ambient conditions have been met.
Other Functions
Perma Pure supported another OEM as they reengineered their existing solution to make dryer replacement easier for the end-user. Within the OEM’s parameters, Perma Pure Additionally, we explore the use of Nafion™ tubing for humidification in medical applications. Too little humidity can impact sensor performance and affect patient comfort. Using Nafion™ tubing to humidify means using similar properties in reverse. If there is more moisture surrounding the membrane than inside the membrane, moisture can be added to the gas stream.
What is the value in controlling humidity with ME Dryers?
The ME Dryer helps create the ideal conditions for the breath or gas sample and helps protect your device from moisture, which in turn, gives your clinicians what they need to care for their patients. The benefits of using ME Dryers aid in maintaining high component standards across your device, reducing inefficiencies across supply, and accessing geographic markets. The benefits include:
- FDA 510K Cleared
- FDA Registered Facility
- ISO 13485 Certified
- ISO 9001 Certified
- CE Marked (EU MDR GSPR)
- Produced in an ISO Class 8 Clean Room
- 100% Leak and Flow Tested
Your goal is to help clinicians monitor, diagnose, care for patients. Perhaps, your device does this by assessing a patient’s exhaled CO2 levels or by measuring a therapeutic gas to ensure the patient is receiving an amount that is neither toxic, nor ineffective. Both applications require a dry sample with a controlled amount of humidity. With the ME Dryers, you are equipping your product with a membrane that supports consistent moisture reduction and meets key regulatory standards across respiratory markets.
How do ME Dryers compare to alternative drying solutions?
ME Dryers are non-porous and will continuously transfer water vapor so long as the ion channels remain unclogged and there is sufficient ambient air surrounding the membrane. With a filter, collecting water vapor becomes less efficient as the filter becomes more saturated. With a water trap, moisture is collected, however, the water trap must be emptied by someone once full. ME Dryers differ from the alternatives and provide an optimal method of controlling moisture in respiratory devices.
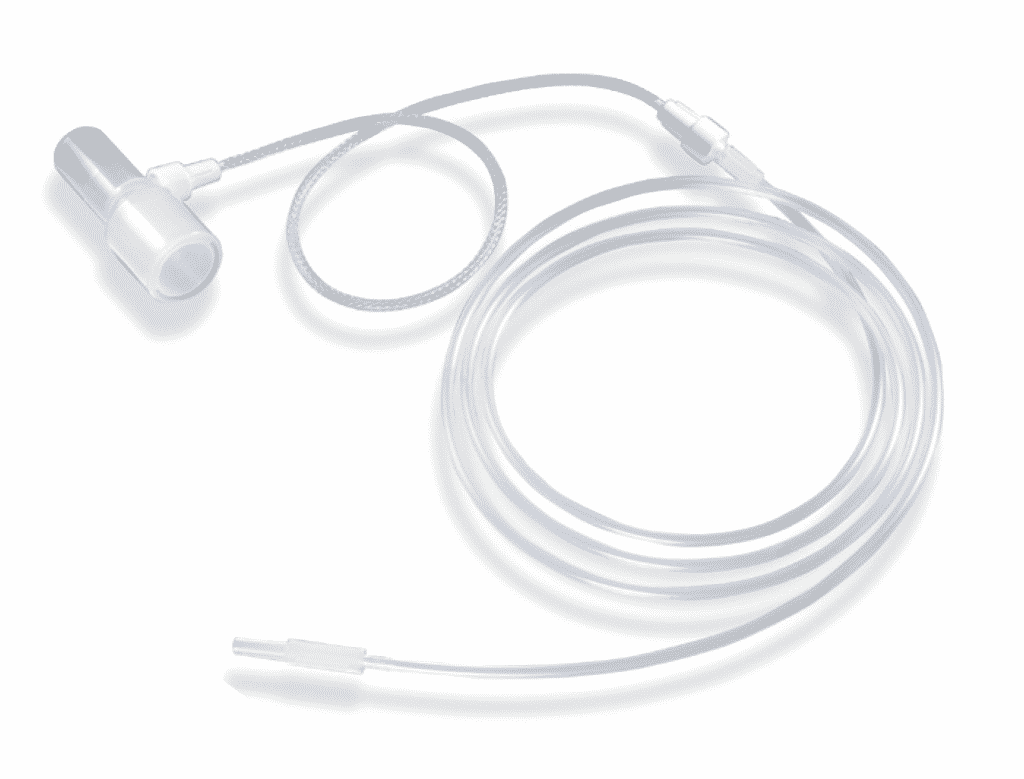
What is the benefit of working with Perma Pure?
Our expertise in moisture control and 50-year history in this space compliments our unique portfolio. In our medical sector, we work with brilliant OEMs, integrating ME Dryers to suit changing device and market needs. Knowing that controlling humidity with Nafion™ tubing has helped create ground-breaking solutions, pushes us to continuously expand our capabilities and optimize how we support our customers. This means understanding how you want to help patients, thinking about how Nafion™ tubing can address moisture concerns in your product, understanding the regulatory impact of adding our dryers, providing customizations and private labeling, and manufacturing components and assemblies based on your specific device requirements. As humidity control experts, we aim to discover how Nafion™ tubing can be most impactful in your device.
To learn more about our ME Dryers and Nafion™ tubing, please visit our website at https://www.permapure.com/medical/ or reach out to our team at info@permapure.com or 1-732-244-0010.
Note: Nafion™ and any associated logo is a trademark or copyright of THE CHEMOURS COMPANY FC, LLC USED UNDER LICENSE BY PERMA PURE LLC.
